Billing Codes for Monoclonal Antibody COVID-19 Infusion
The FDA issued an EUA on November 21, 2020, for the investigational monoclonal antibody therapy, casirivimab and imdevimab, administered together to treat the adult and pediatric patients with mild-to-moderate COVID-19 having positive COVID-19 test results high at risk for progressing to severe COVID-19 and/or hospitalization. The administration can only occur in settings where healthcare providers have immediate access to medications to treat a severe infusion reaction and the ability to activate the emergency medical system (EMS), as necessary.
During the COVID-19 public health emergency (PHE), Medicare ensures coverage and payment for these infusions the same way it does for COVID-19 vaccines, thus allowing coverage for a broad range of providers and suppliers, including home health agencies, nursing homes, and freestanding and hospital-based infusion centers, and entities with whom nursing homes contract for this, to administer these treatments. Medicare will refuse payment for the COVID-19 monoclonal antibody products that providers receive for free. If providers begin to purchase COVID-19 monoclonal antibody products, Medicare anticipates setting the payment rate for the products, which will be 95% of the average wholesale price (AWP) for many healthcare providers, consistent with the usual vaccine payment methodologies. Medicare soon anticipates establishing codes and rates for the administration of the products.
CMS recognized specific code(s) for each COVID-19 monoclonal antibody product and specific administration code(s) for Medicare payment:
EUA effective November 10, 2020, for Eli Lilly and Company’s Antibody Bamlanivimab (LY-CoV555)
Healthcare providers can now use the HCPCS code Q0239 for the injection of 700 mg of Eli Lilly and Company’s investigational monoclonal antibody therapy cocktail and code M0239 for intravenous infusion and post-administration monitoring, according to CMS source on Monoclonal Antibody COVID-19 Infusion
EUA effective November 21, 2020, for Regeneron’s Antibody casirivimab and imdevimab (REGN-COV2) (ZIP)
Healthcare providers can now use the HCPCS code Q0243 to inject 2,400 mg of Regeneron’s investigational monoclonal antibody therapy cocktail and code M0243 for intravenous infusion post-administration monitoring, according to CMS source on Monoclonal Antibody COVID-19 Infusion
Get the latest and most up to date list of billing codes, payment allowances, and effective dates.
Payment for Product & Infusion
Medicare will not provide payment for the COVID-19 monoclonal antibody products that healthcare providers receive for free, as will be the case upon the product’s initial availability in response to the COVID-19 PHE. If healthcare providers begin to purchase these monoclonal antibody products, CMS foresees setting the payment rate in the same way it was addressed the rate for COVID-19 vaccines.
In order to ensure immediate access during the COVID-19 PHE, Medicare will cover and pay for these infusions per Section 3713 of the Coronavirus Aid, Relief, and Economic Security Act (CARES Act). CMS proposes to address potential refinements to payment for COVID-19 monoclonal antibody infusions and their administration through future notice and comment rulemaking.
Initially, the Medicare national average payment rate for the administration will be $309.60; this payment rate is strictly based on one hour of infusion and post-administration monitoring in the hospital outpatient setting. In the future, CMS may utilize a similar methodology to determine the payment rate for the infusion of additional monoclonal antibody products based on the expected infusion time in line with the FDA EUA or FDA approval of such products.
CMS is hopeful that this Medicare coverage for the two therapies will help providers overcome the hurdles of acquiring and employing new treatments. Yet, it is a known fact that healthcare facilities and providers are still expected to encounter challenges obtaining an adequate supply of such therapies for infected patients.
Optimize your operational efficiency and revenue with our state of art RCM Technology Platform. Schedule an online demonstration now.
The FDA issued an EUA on November 21, 2020, for the investigational monoclonal antibody therapy, casirivimab and imdevimab, administered together to treat the adult and pediatric patients with mild-to-moderate COVID-19 having positive COVID-19 test results high at risk for progressing to severe COVID-19 and/or hospitalization. The administration can only occur in settings where healthcare providers have immediate access to medications to treat a severe infusion reaction and the ability to activate the emergency medical system (EMS), as necessary.
During the COVID-19 public health emergency (PHE), Medicare ensures coverage and payment for these infusions the same way it does for COVID-19 vaccines, thus allowing coverage for a broad range of providers and suppliers, including home health agencies, nursing homes, and freestanding and hospital-based infusion centers, and entities with whom nursing homes contract for this, to administer these treatments. Medicare will refuse payment for the COVID-19 monoclonal antibody products that providers receive for free. If providers begin to purchase COVID-19 monoclonal antibody products, Medicare anticipates setting the payment rate for the products, which will be 95% of the average wholesale price (AWP) for many healthcare providers, consistent with the usual vaccine payment methodologies. Medicare soon anticipates establishing codes and rates for the administration of the products.
CMS recognized specific code(s) for each COVID-19 monoclonal antibody product and specific administration code(s) for Medicare payment:
EUA effective November 10, 2020, for Eli Lilly and Company’s Antibody Bamlanivimab (LY-CoV555)
Healthcare providers can now use the HCPCS code Q0239 for the injection of 700 mg of Eli Lilly and Company’s investigational monoclonal antibody therapy cocktail and code M0239 for intravenous infusion and post-administration monitoring, according to CMS source on Monoclonal Antibody COVID-19 Infusion
EUA effective November 21, 2020, for Regeneron’s Antibody casirivimab and imdevimab (REGN-COV2) (ZIP)
Healthcare providers can now use the HCPCS code Q0243 to inject 2,400 mg of Regeneron’s investigational monoclonal antibody therapy cocktail and code M0243 for intravenous infusion post-administration monitoring, according to CMS source on Monoclonal Antibody COVID-19 Infusion
Get the latest and most up to date list of billing codes, payment allowances, and effective dates.
Payment for Product & Infusion
Medicare will not provide payment for the COVID-19 monoclonal antibody products that healthcare providers receive for free, as will be the case upon the product’s initial availability in response to the COVID-19 PHE. If healthcare providers begin to purchase these monoclonal antibody products, CMS foresees setting the payment rate in the same way it was addressed the rate for COVID-19 vaccines.
In order to ensure immediate access during the COVID-19 PHE, Medicare will cover and pay for these infusions per Section 3713 of the Coronavirus Aid, Relief, and Economic Security Act (CARES Act). CMS proposes to address potential refinements to payment for COVID-19 monoclonal antibody infusions and their administration through future notice and comment rulemaking.
Initially, the Medicare national average payment rate for the administration will be $309.60; this payment rate is strictly based on one hour of infusion and post-administration monitoring in the hospital outpatient setting. In the future, CMS may utilize a similar methodology to determine the payment rate for the infusion of additional monoclonal antibody products based on the expected infusion time in line with the FDA EUA or FDA approval of such products.
CMS is hopeful that this Medicare coverage for the two therapies will help providers overcome the hurdles of acquiring and employing new treatments. Yet, it is a known fact that healthcare facilities and providers are still expected to encounter challenges obtaining an adequate supply of such therapies for infected patients.
Optimize your operational efficiency and revenue with our state of art RCM Technology Platform. Schedule an online demonstration now.

Get Updated with Latest News
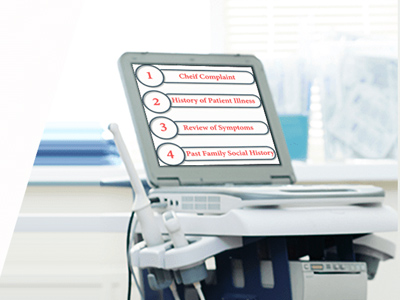
Telehealth and Covid-19: 2020 Coding & Billing Tips
Telehealth and COVID-19: 2020 Coding and Billing Tips
Optimize your operational efficiency and revenue with our state of art RCM Technology Platform. Schedule an online demonstration now.

Get Updated with Latest News

2020 Annual Wellness Visit (AWV) Coding and Documentation Tips
2020 Annual Wellness Visit (AWV) Coding and Documentation Tips
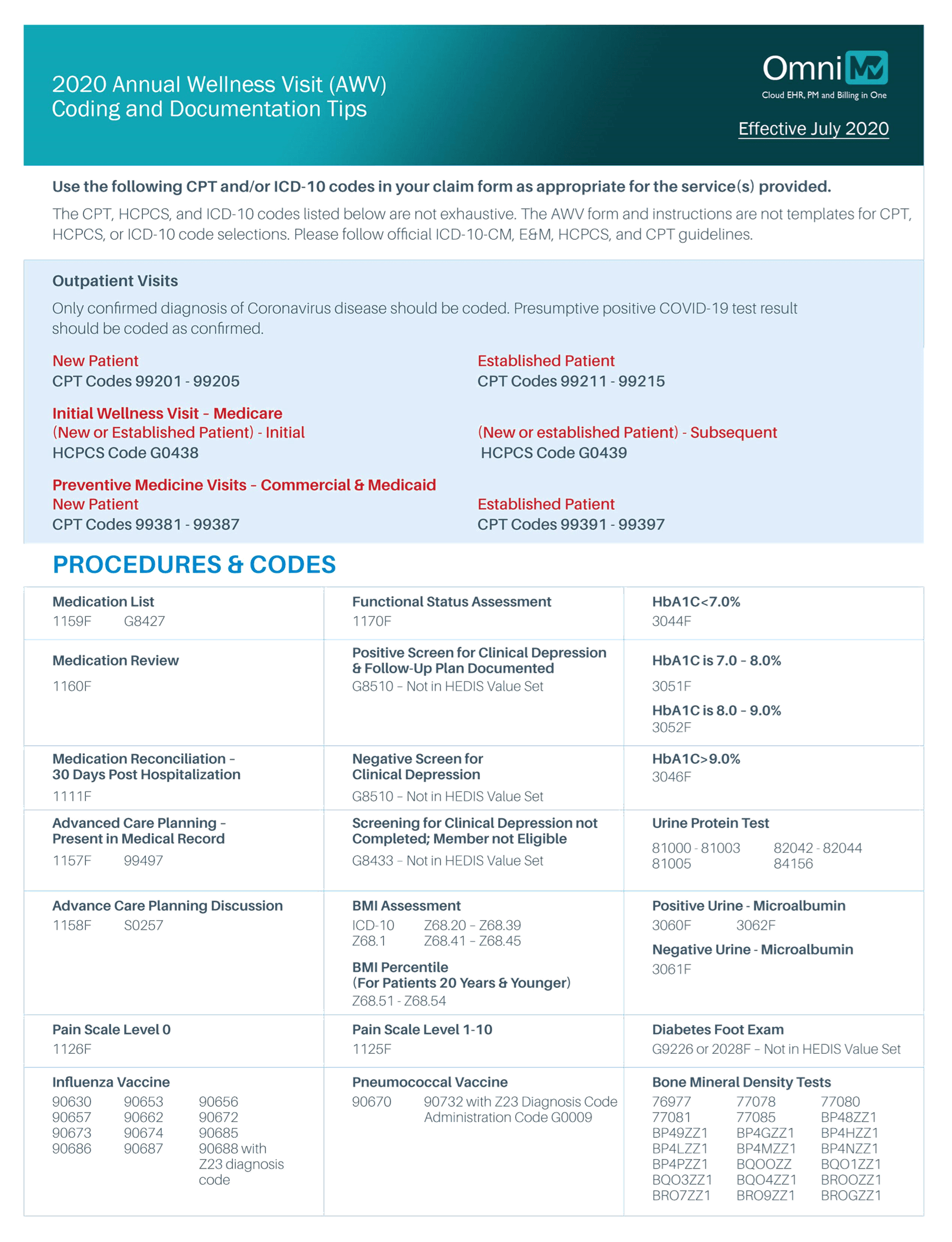
2020 Annual Wellness Visit (AWV) Coding and Documentation Tips
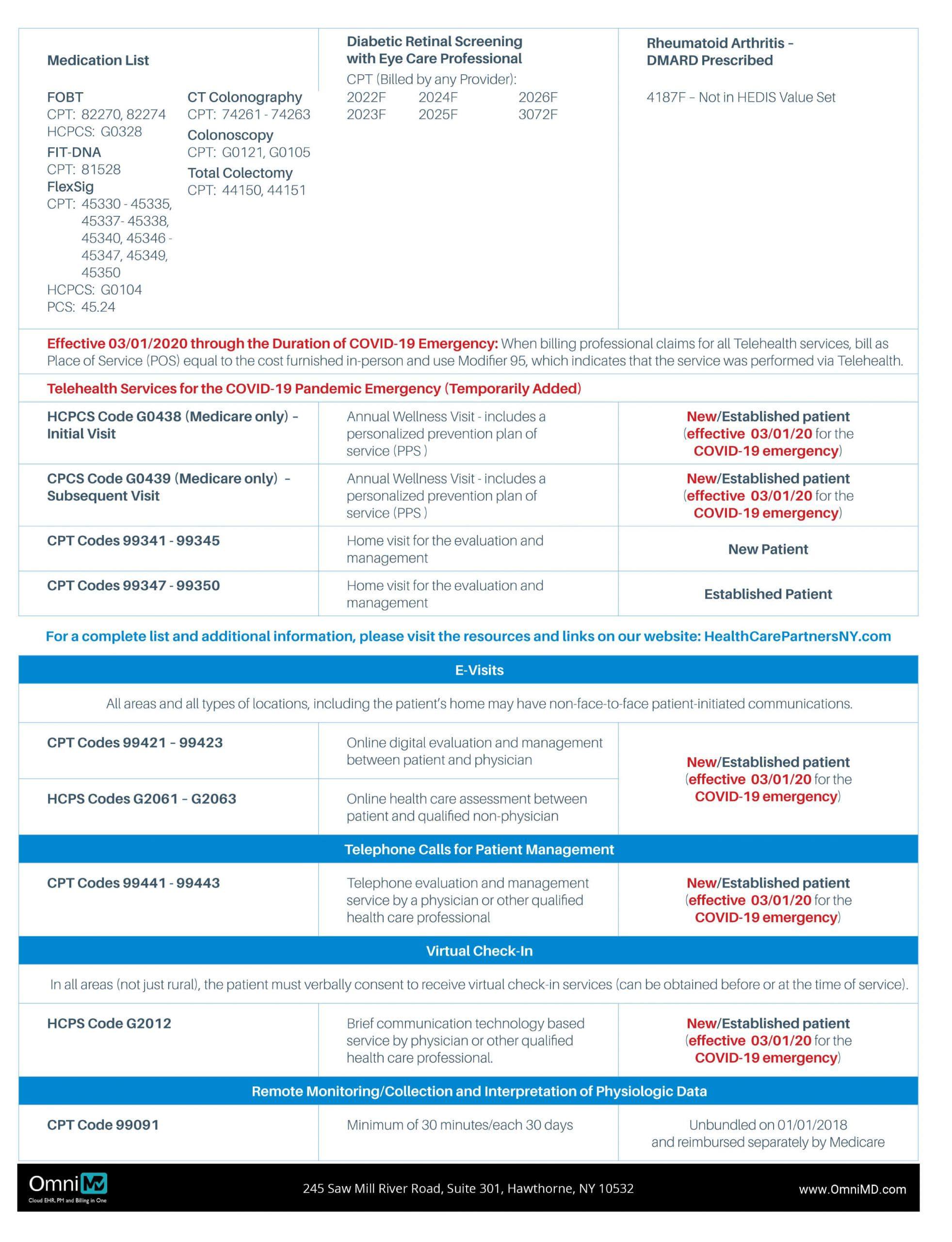
Coding and Documentation Tips

Get Updated with Latest News

The Ultimate Guide to Revised E&M Guidelines for Office and Other Outpatient Services in 2021
The Ultimate Guide to Revised E&M Guidelines for Office and Other Outpatient Services in 2021
Centers for Medicare and Medicaid Services (CMS) has embraced the recommendations of the AMA in regards to Evaluation and Management (E&M). Starting January 1, 2021, these changes will be applicable for coding office and other outpatient services.
Reason
In 2017, CMS brought an initiative “Patients Over Paperwork” to streamline work, increase efficiency, improve patient experience, and reduce administrative burden.
The purpose of this initiative was to revise the existing and archaic E&M coding guidelines. According to CMS, increasing paperwork and reporting tools were the main bottlenecks that kept physicians and medical practices busy.
They were required to spend more time managing administrative tasks instead of caring for patients. It resulted in poor patient experience and monumental administrative tasks that added to the cost as physicians needed to hire additional staff and comply with the government rules and regulations.
What Changes Will Take Effect
History and Tests Removed as Mandatory Elements for Coding. These two components tend to delay clinical decision making and are time-consuming as the clinicians need to document this in the patients’ medical record.
Documents related to Medical Decision Making or Time
Physicians cannot use both these documentation methods for the same patient visit. They need to select either option for each patient visit.
Updated Time-Based Coding
Time is explained as “total time on the date of the encounter.” It includes time spent by authorized healthcare professionals and clinicians for in-person and other modes or non-face-to-face discussions with the patient.
Revised Coding for Prolonged Services
It should be used for time-based coding when the duration of the encounter exceeds the defined time for 99205 and 99215 in 15-minute increments.
Updated MDM Criteria
Using the present CMS Table of Risk as a standard guideline, the MDM elements for code selection were refined and clarified to avoid complexity and increase efficiency patient management.
Restructuring of RVUs and Charges
Considering the RVU guidelines from the AMA’s CPT/RUC Workgroup on E&M, CMS has stated that relative value for the codes is evaluated depending on the total duration spent by a physician from three days before patient’s visit through seven days following the visit as the standard work will be same irrespective of the time when it is completed.
Source: https://www.ama-assn.org/
Optimize your operational efficiency and revenue with our state of art RCM Technology Platform. Schedule an online demonstration now.